Bringing a medical device from concept to reality has never been more difficult. Manufacturers must strike a balance between rigorous regulatory standards, complicated product variations, and rising customer demands. In this scenario, the ‘medical device time to market’ process becomes a critical competitive component. Even minor delays in the process can lead to revenue loss and poor customer experience, while creating opportunities for competitors.
In this ‘medical device time to market’ process, most enterprises concentrate on design, engineering, and compliance. Though they are extremely important, another critical component sometimes goes unnoticed: the sales and quotation process. Inefficiencies in configuration, price, documentation, and approvals can quietly push the entire timeline out by weeks or even months. In a competitive market, that difference can be substantial.
This is where medical device CPQ systems make a measurable difference. They simplify one of the most fragmented parts of the process and remove the friction that typically slows everything else down.
Time-to-Market Is a Competitive Advantage and Also a Significant Challenge
Every medical device manufacturer wants to move faster, but the journey from product concept to market release is full of hurdles. A small error in the configuration can cause a disruption, delaying production schedules and slowing the path to regulatory submissions. Many organizations accept these delays as unavoidable. However, the truth is that some hurdles are the result of outdated tools and disconnected processes. As a result, they can be fixed easily.
For medical device manufacturers, every step needs to be accurate. Documentation must be accurate. Every optional component must be validated. Every configuration must meet engineering rules and regulatory expectations.
To achieve this, a growing number of manufacturers are now using medical device CPQ platforms. Not because CPQ solves engineering or regulatory challenges but how it prevents small errors and misalignments in the sales process from turning into bigger delays later.
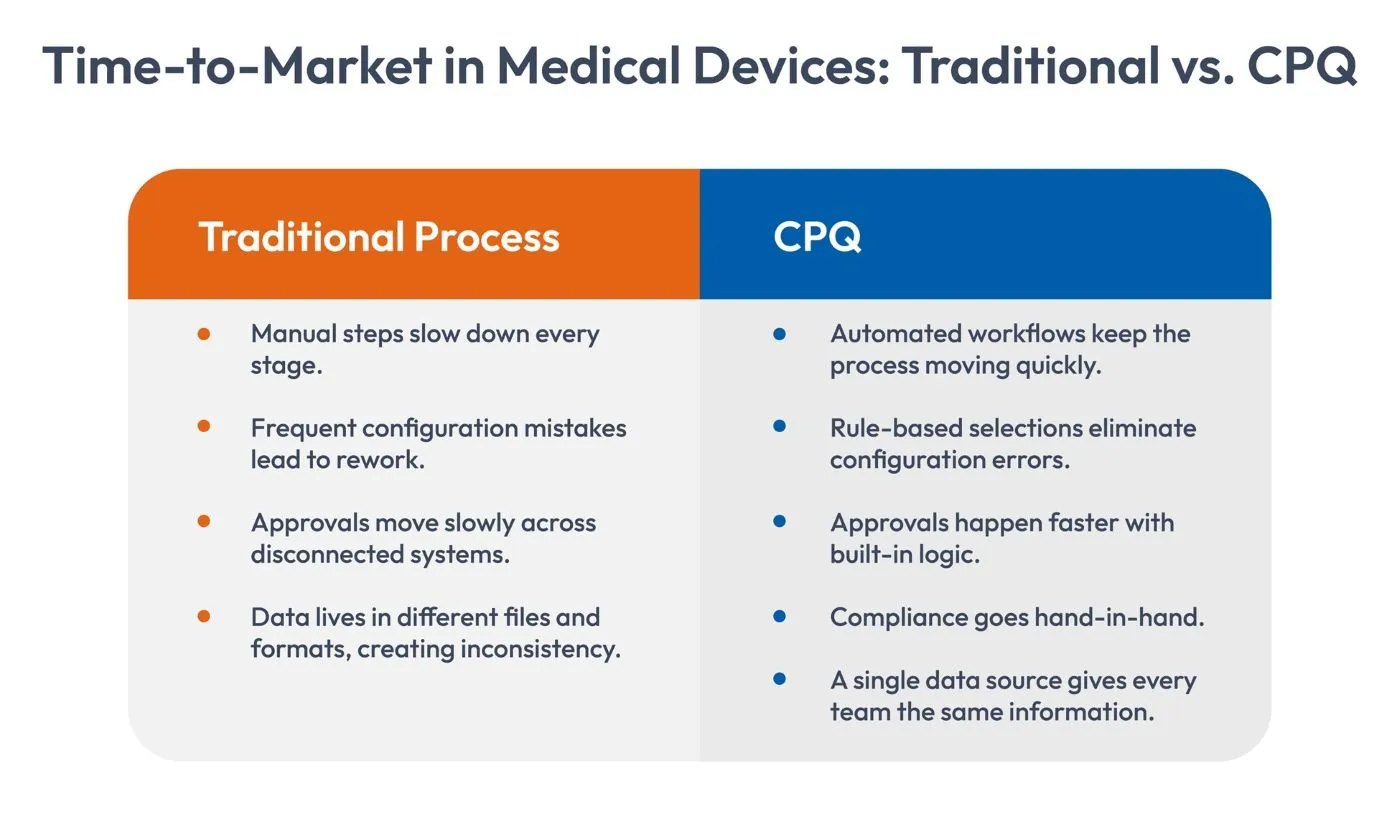
How CPQ Helps Reduce Time-to-Market
A modern CPQ system doesn’t replace engineering tools or regulatory systems. Instead, it complements them. It becomes the place where the most critical details, including product rules, configurations, pricing, documentation needs, and approval thresholds are brought together.
Accurate Product Configuration
When sales teams use CPQ, they don’t guess. They don’t rely on old spreadsheets. They don’t choose incompatible components. They simply answer guided questions, select valid options, and receive an accurate configuration that meets engineering constraints and regulatory expectations.
CPQ ensures that only valid combinations are selected. Any incompatible selections are automatically blocked. This single change reduces the risk of rework more than anything else. This benefits the entire organization:
- Engineering no longer needs to re-validate basic configurations
- Documentation teams always receive the right details
- Operations avoid last-minute changes to the BOM
- Sales can respond to customers much faster
When configuration errors disappear, so does the rework that often interrupts production planning.
Faster Quote Generation
For medical device manufacturers, the quote preparation and approval process is amongst the longest and time-taking processes as it involves multiple departments.
CPQ simplifies this by placing all approval rules inside the system. A quote is automatically routed to the right person, with complete context and supporting details attached. Approvers don’t search through emails or documents. They see everything they need in one place and approve quickly.
Clean Sales-to-Engineering Handoff
When the engineering team receives incomplete specs or invalid combinations, they have to pause their work and clarify the details. This back-and-forth causes small but persistent delays. CPQ removes this friction entirely. Once a configuration is completed, engineering receives:
- a validated, rules-driven configuration
- a complete and accurate BOM
- correct accessory and module selections
- pricing aligned with the commercial offer
- specification details needed for documentation
A cleaner handoff enables engineering teams to begin planning and resource allocation sooner, which directly contributes to a shorter ‘medical device time to market’ cycle.
Integration with ERP and PLM Systems
When CPQ is connected with ERP and PLM systems, manufacturers gain a true end-to-end workflow with no manual handoffs or data inconsistencies. Every approved product configuration flows directly into production planning. Pricing, customer data, and order details remain accurate from the quote to production cycle. All of this helps teams work with up-to-date data, eliminating guesswork and manual updates.
Better Planning
One benefit many leaders don’t expect from CPQ is improved forecasting. When all quotes are created through a single platform, organizations suddenly gain a clear view of upcoming demand. They can see which devices are trending, which configurations are becoming more common, and which regions are generating growth.
This visibility helps teams:
- prepare production schedules earlier
- plan component inventory more accurately
- allocate resources for high-demand modules
- avoid last-minute procurement delays
Why Many Medical Device Manufacturers Choose Cincom CPQ
As medical device portfolios grow more complex, manufacturers need a CPQ platform that not only handles configurations but also connects the entire quoting workflow. This is where Cincom CPQ fits naturally into the process. It brings together configuration logic, pricing intelligence, documentation needs, and approval rules into one unified system.
It supports detailed product rules and multi-level components, while providing accurate 2D and 3D designs for the final product. It also connects seamlessly with leading CRM and ERP systems, ensuring every team, from sales to engineering to operations, has access to accurate and real time data, and the entire process runs smoothly.
Helmer Scientific, a manufacturer of specialized medical devices and laboratory equipment, chose Cincom to optimize their sales and order management processes. They integrated Cincom CPQ with their Microsoft Dynamics CRM and Fourth Shift ERP to improve accuracy in quotes, streamline order management, and enhance system scalability.
Conclusion
In an industry where competition is increasing and regulatory expectations are rising, speed matters. Even modest reductions in delay can give medical device manufacturers a meaningful market advantage. CPQ doesn’t replace the innovation that happens in R&D or the critical oversight provided by regulatory teams, but it does strengthen the connective layers between sales, engineering, documentation, and operations.
For enterprises looking to improve ‘medical device time to market’ cycle, CPQ is one of the simplest and the most effective changes they can make. It brings clarity where there is confusion, accuracy where there is risk, and speed where delays were once normal.
Small improvements add up. And in the medical device world, the right small change can be the difference between entering the market on time or falling behind.
FAQs
1. How does CPQ improve the accuracy of medical device configurations?
CPQ uses built-in product rules and guided selections. It prevents incompatible options, ensures compliance-friendly configurations, and removes guesswork from the sales process.
2. How does CPQ reduce rework during production planning?
CPQ reduces rework by validating product configurations upfront. When engineering receives clean, accurate configuration, the need for clarifications and corrections drops significantly.
3. What happens when CPQ integrates with ERP and PLM?
When CPQ integrates with ERP and PLM solutions, manufacturers gain a connected workflow. Data flows seamlessly from quote to manufacturing planning. Teams work from the same accurate information without manual updates.
4. Can CPQ speed up the approval process?
Yes. CPQ routes quotes automatically based on approval rules. Approvers get all details in one place, which helps them review and approve much faster.
5. Does CPQ replace engineering or regulatory systems?
No. CPQ doesn’t replace engineering tools or regulatory platforms. It complements them by ensuring that the sales process doesn’t introduce errors that slow down those teams.




