Accuracy, compliance, and traceability are the three crucial pillars in the medical device industry. However, data discrepancies and compliance gaps become nearly unavoidable when sales, engineering, quality, and manufacturing function in disconnected systems. The industry as a whole is becoming more aware of these problems. Medical device recalls hit a four-year high in 2024 with 1,059 recall occurrences, according to Sedgwick’s 2025 U.S. Recall Index.
Errors in software and documentation account for a large percentage of these recalls. Software design flaws alone were responsible for 32%–42% of recalls in AI/ML-enabled medical devices, as per JMIR Medical Informatics. This shows how crucial data quality and controlled setup are to compliance.
CPQ plays a pivotal role to cater to challenges like this. CPQ-CRM-ERP integration guarantees uniform data flow, enforces regulatory standards, and offers full traceability across every quote, configuration, and approval. Manufacturers can drastically reduce the mistakes that result in recalls. How? Take a look at the blog.
Why Data Integrity Is Critical in the MedTech Industry
Medical device manufacturers operate in one of the world’s most highly regulated environments, governed by FDA, ISO 13485, EU MDR, and other global standards. Every customer quote, configuration, discount, and approval becomes part of the official device history and must withstand regulatory scrutiny.
Poor data integrity can lead to:
- Incorrect configurations triggering non-compliant builds
- Missing documentation required during audits
- Inaccurate pricing or component selection
- Compliance gaps due to manual data entry
- Delayed approvals and slow time-to-quote
This is why solutions that protect data accuracy, like integrated CPQ, play a critical role in supporting medical device regulatory compliance.

Integrating CPQ with ERP and CRM Systems: A Roadmap for Sales and Operations Leaders
Understanding the Role of CPQ in Medical Device Sales
CPQ software ensures that every quote generated by sales aligns with engineering rules, quality requirements, and regulatory standards. CPQ serves as both a regulated compliance system and a quotation tool.
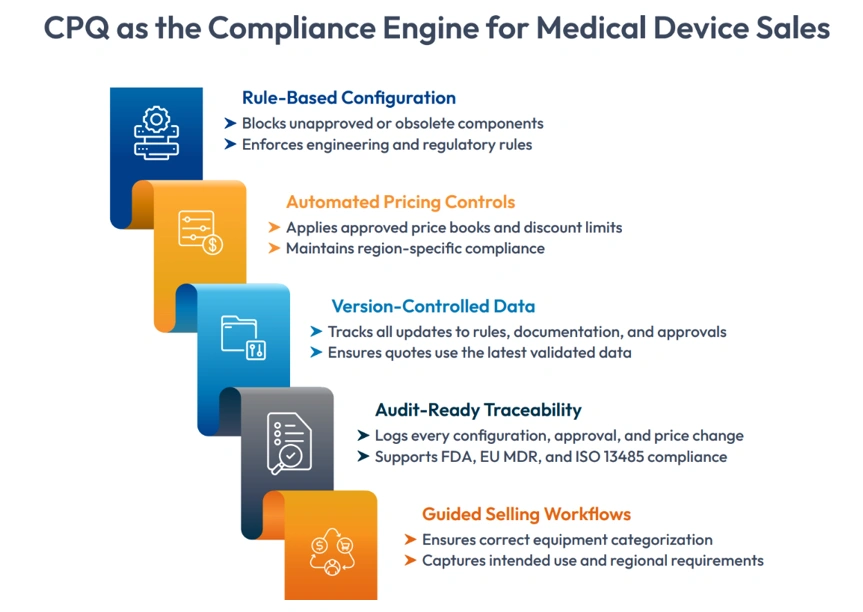
Important capabilities are:
1. Rule-based Configuration
CPQ enforces engineering and regulatory guidelines to prohibit sales from choosing obsolete components, unapproved options, or combinations that violate design specifications.
2. Automated Pricing Controls
This lowers the possibility of unauthorized pricing by automatically aligning prices with approved discount levels, validated cost data, and region-specific compliance requirements.
3.Version-Controlled Data
CPQ ensures that quotations always utilize the most recent verified data by strictly versioning configuration rules, product documentation, regulatory comments, and approvals.
4. Audit-Ready Traceability
All changes are automatically logged, including configuration adjustments, approvals, discounts, and attachments. This produces the whole digital trail needed for EU MDR, ISO 13485, and FDA audits.
5. Guided Selling Workflows
Sales representatives adhere to established question flows that guarantee proper recording of equipment categorization, intended usage information, and region-specific needs.
When combined, these features allow CPQ to serve as a single point of truth for engineering, sales, and quality teams. Every quotation, configuration, and approval stage complies with medical device regulatory compliance criteria due to this centralization.
How CPQ Integrates Seamlessly with CRM and ERP Systems
When CPQ is connected with CRM and ERP, all teams work from the same data—removing silos and preventing quoting or production errors.
How the Integration Works?
1. CRM → CPQ
CRM passes customer and commercial data into CPQ, including:
- Account details and buying history
- Contract terms and approved price lists
- Customer-specific discounts
- Installed base or renewal details
Result: CPQ generates accurate, customer-specific quotes without manual entry.
2. CPQ → ERP
CPQ sends production-ready data to ERP after approval:
- Configured product specifications
- Final pricing and margin details
- Bill of materials (BOM)
- Routing or resource requirements
Result: ERP begins production planning, inventory checks, and procurement immediately.
3. ERP → CPQ
ERP returns real-time operational updates to CPQ:
- Component availability
- Latest material and production costs
- Lead times and capacity constraints
Result: Quotes reflect actual supply chain, cost, and production conditions.
Unified Audit Trail
- CPQ tracks configuration and pricing actions
- CRM validates customer and commercial data
- ERP verifies inventory, cost, and production data
Result: Complete traceability for compliance—critical for regulated industries like medical devices.
Why It Matters for MedTech
- Prevents configuration and pricing errors
- Ensures quotes match engineering, cost, and supply realities
- Eliminates manual data transfer between systems
- Reduces audit and compliance risks
Ensuring Medical Device Regulatory Compliance and Traceability Through Integration
Regulators need total transparency on the configuration, pricing, approval, and manufacturing of a product. Every step is tracked, verified, and connected when CPQ is integrated with CRM and ERP, resulting in a process that is completely auditable.
Key Compliance Features Enabled Through Integration
1. Accurate Audit Trails for Medical Device Quoting
- CPQ logs every configuration choice, rule trigger, discount, and approval with timestamps.
- Ensures full visibility during FDA, MDR, or ISO audits.
2. End-to-End Traceability
- Customer data → configuration → pricing → production details flow automatically across CRM, CPQ, and ERP.
- Eliminates manual data edits that cause compliance gaps.
3. Controlled Configuration Rules
- Only approved product combinations and variants can be selected.
- Prevents non-compliant device builds and configuration errors.
4. QMS Alignment
- CPQ connects to QMS-controlled processes such as design controls, CAPA, and document management.
- Ensures configurations match the latest approved engineering and quality documentation.
5. Regulatory Document Linkage
- IFUs, labeling rules, certification files, and component standards are linked directly to the configured product.
- Ensures quotes and builds align with current regulatory documentation.
Benefits of Unified Data Flow Across Sales, Production, and Quality
When CPQ is integrated with CRM and ERP, manufacturers experience improvements across the entire value chain:
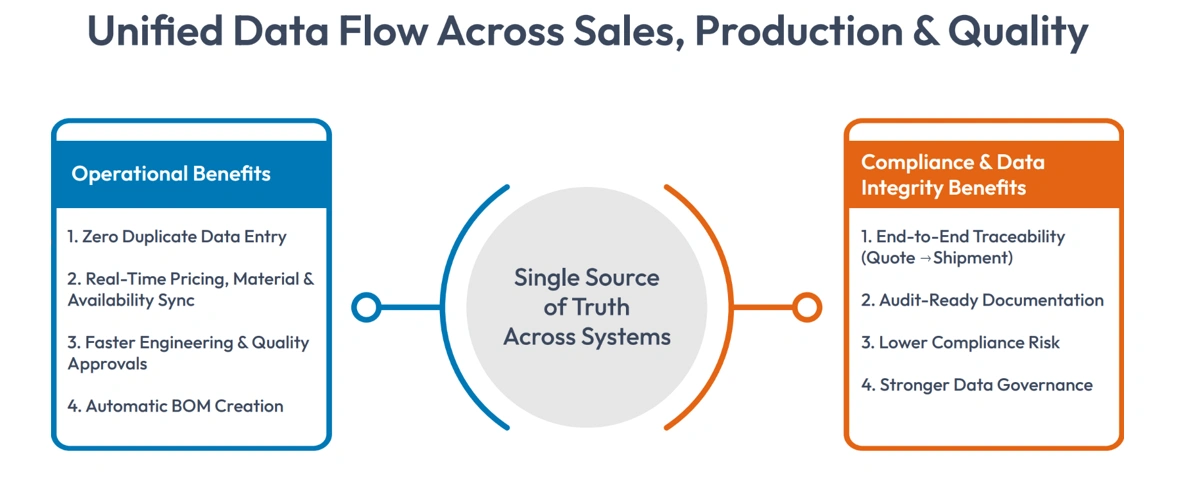
Operational Benefits
- Zero duplicate data entry
- Real-time synchronization of pricing, materials, and availability
- Faster approvals from engineering and quality teams
- Automatic BOM creation based on compliant configurations
Compliance and Data Integrity Benefits
- Complete traceability from quote to shipment
- Audit-ready documentation at every sales stage
- Reduced compliance risk associated with manual processes
- Stronger data governance across systems
Together, these benefits safeguard data integrity and ensure that every product delivered complies with regulatory standards.

Find out how this medical device manufacturer benefited from its partnership with Cincom CPQ.
Best Practices for Implementing CPQ in Regulated Manufacturing Environments
1. Build Compliance-First Configuration Rules
Use only product families, authorized components, and configuration logic that complies with regulations.
2. Integrate CPQ with CRM and ERP Early
For medical processes, early integration guarantees continuous data flow and enhances CPQ–CRM–ERP data integrity.
3. Maintain Documented Version Control
To ensure traceability, keep track of any changes made to price, product information, and configuration rules.
4. Align CPQ with QMS Workflows
Link CPQ to quality review, document management, design controls, and change control procedures.
5. Implement Audit Trail Monitoring
Verify that configuration, pricing, and approval activities are completely audit-ready by routinely reviewing logs.
6. Train Sales and Engineering Teams
Make sure teams are aware of authorized configurations, legal requirements, and the consequences of wrong selection.
Conclusion
CPQ integration with CRM and ERP is an absolute mandate for medical device manufacturers. It ensures medical device regulatory compliance, maintains robust data integrity, and delivers traceability across the entire quote-to-manufacture lifecycle. Manufacturers lower risk, speed up sales cycles, and confidently produce high-quality devices with unified data, automated workflows, and compliance-ready audit trails.
Want to transform your quoting process with compliance-first automation? Schedule a demo now!
FAQs
1. How does CPQ help ensure compliance in medical device manufacturing?
CPQ enforces controlled configuration rules, prevents non-approved product combinations, and ensures every quote follows validated engineering, pricing, and regulatory standards. It also links required documents—such as IFUs, certifications, and labeling rules—to each configured product.
2. Why is data integrity important in regulated industries?
Regulated industries depend on accurate, consistent data to meet safety, quality, and regulatory requirements. Any incorrect or manually altered data can lead to non-compliant builds, reporting errors, audit failures, and potential regulatory penalties.
3. What are the benefits of integrating CPQ with CRM and ERP systems?
Integration creates a unified data flow across sales, engineering, production, and quality. This eliminates duplicate data entry, ensures real-time visibility into pricing and component availability, automates BOM creation, strengthens compliance, and improves quoting accuracy.
4. Can CPQ help maintain audit trails and traceability for compliance audits?
Yes. CPQ logs every configuration decision, pricing change, approval, and rule trigger with timestamps. When connected with CRM and ERP, it forms a complete traceability chain from customer input to production, supporting regulator-ready audit documentation.
5. What best practices should MedTech manufacturers follow for CPQ implementation?
Manufacturers should build compliance-first configuration rules, integrate CPQ with CRM and ERP early, maintain strict version control, align CPQ with QMS processes, monitor audit logs regularly, and train sales and engineering teams on regulatory dependencies.



