Introduction
According to McKinsey & Company, the MedTech industry is expected to reach $600 billion, with 5–6% annual growth projected through 2026. This growth is fueled by expanded access to care, increased demand for personalized solutions, and ongoing innovation across the sector.
Amidst this growth, medical device manufacturers face mounting pressures:
- Accelerate time to market
- Customize complex, configure-to-order products
- Comply with strict regulatory requirements (e.g., FDA, EU MDR)
- Compete in an increasingly globalized market
Yet, many companies continue to rely on manual quoting processes or outdated systems. These methods slow down the sales cycle, introduce costly errors, and increase compliance risk. Sales teams struggle to configure specialized devices, pricing remains inconsistent, and delays in approvals often lead to lost deals.
A modern quoting tool for medical devices, especially one like Cincom CPQ built for configure-to-order (CTO) scenarios, addresses these challenges. It streamlines configuration, pricing, and proposal creation, ensuring every quote is accurate, compliant, and delivered quickly.
Discover How a Quoting Tool Can Revolutionize Your Medical Device Sales. Request a Demo
Here are the top five reasons to implement a quoting tool for medical devices in 2025.
1. The Cost of Inaccuracy in Medical Device Quoting
In the medical device industry, accuracy isn’t just a sales concern—it’s a compliance and patient safety issue.
Common quoting risks:
- Misconfigured devices leading to regulatory violations
- Delayed approvals due to incomplete documentation
- Product recalls caused by incorrect specifications
Manual quoting compounds these issues:
- Spreadsheets lack built-in compliance checks
- Systems don’t enforce FDA, EU MDR, or regional rules
- Errors often go unnoticed until late in the sales process
How quoting tools reduce compliance risk:
- Built-in rules restrict configurations to only approved options
- Automatic validation against clinical and regulatory requirements
- Flags issues before quotes reach customers
- Prevents incomplete or non-compliant offerings

How CPQ Helps Manufacturers Protect Margins and Increase Sales
Download the whitepaper to see how CPQ boosts sales, protects profits, and adapts quickly to market changes.
2. Faster Turnaround Times Drive Sales Wins
In medical sales, delays can be deal-breakers. Slow quote turnaround increases the risk of lost opportunities and customer churn.
Manual Quoting vs. CPQ-Enabled Quoting
| Feature | Manual Quoting | CPQ-Enabled Quoting |
| Quote Turnaround Time | Days or weeks | Minutes to hours |
| Configuration Accuracy | High risk of errors | Rules-based, guided |
| Data Entry | Manual, repetitive | Automated, integrated |
| Compliance Checks | Manual review | Real-time validation |
| Proposal Generation | Inconsistent | On-brand, instant |
| Sales Cycle Impact | Slower | Faster, higher win rate |
How quoting tools speed up sales:
- Reps can configure and price devices live during customer meetings
- Pre-built templates generate proposals instantly
- CRM/ERP integration automates data entry and approvals
Did you know companies using CPQ software report up to 60% faster quote turnaround.
3. Support for Configure-to-Order (CTO) Complexity
CTO products like imaging systems or surgical tools require precise, customized configurations.
Challenges of CTO quoting:
- Complex BOMs and optional accessories
- Variable regional specs (e.g., voltage, labeling)
- Configuration affects pricing, licensing, and compliance
How quoting tools simplify CTO:
- Guided selling prompts reps to gather correct use case info
- Rules ensure only valid part combinations are quoted
- Automatically adjusts for region.
Benefit: Reduces engineering involvement in quote validation, freeing technical teams to focus on product innovation.
4. Better Collaboration Between Sales, Engineering, and Regulatory Teams
Creating quotes for medical devices often involves input from multiple teams—not just sales, but also engineering, product, and regulatory. Without a shared system, collaboration becomes fragmented and inefficient.
How quoting tools improve collaboration:
The system is unified
- Eliminates long email chains and version control issues
- Ensures real-time visibility for all stakeholders
Built-in quote approval workflows
- Automates the review process across teams
- Ensures quotes meet both technical and regulatory standards
- Reduces delays caused by manual handoffs or miscommunication
Engineers can pre-define configuration rules
- Sales reps no longer need to consult technical teams for every quote
- Speeds up quoting and reduces the burden on engineering resources
A centralized quoting system creates smoother collaboration, faster approvals, and fewer errors—keeping your quoting process efficient and compliant.
5. Scalable Growth Through Global and Multi-Channel Quoting
As medical device companies expand into new regions and diversify their sales channels, quoting complexity increases. Whether selling through direct reps, regional distributors, or digital platforms, maintaining quote consistency becomes a challenge.
Manual quoting processes often can’t keep up with:
- Multiple languages
- Country-specific regulations
- Regional pricing strategies
- Diverse partner networks
This is where a modern quoting tool offers real value. It enables scalable, consistent quoting across global markets and sales channels, without the need for heavy IT customization.
Concerned about Regulatory Compliance in Your Quoting? Talk to an Expert
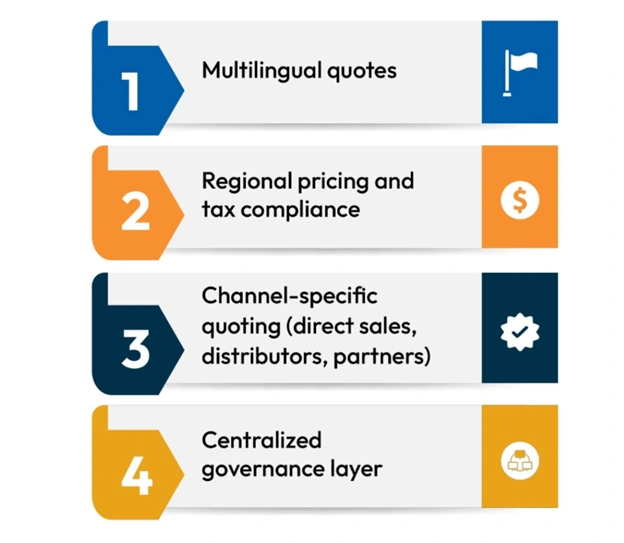
Key capabilities of quoting tools for global scale:
Multilingual quote generation
Create localized, professional proposals in the preferred language of each customer or region.
Regional pricing and compliance rules
Automatically apply the correct tax structures, currencies, and country-specific regulatory requirements.
Centralized control with local flexibility
Headquarters can define global standards, while local reps or partners can adjust within approved parameters.
Partner enablement
Distributors and resellers can generate accurate quotes within the same system, eliminating errors and protecting margins.
Future-proof your quoting process:
- Easily adapt to new product launches, expanded territories, or emerging channels (like eCommerce or virtual sales teams).
- Scale operations without reengineering your entire quoting infrastructure.
A scalable quoting tool supports business growth, improves operational control, and ensures a consistent customer experience—no matter where or how you sell.
Conclusion
In 2025, quoting tools are no longer optional for medical device companies. From reducing compliance risk to accelerating deals and enabling global expansion, a specialized quoting solution is critical for success.
The risks of doing nothing are real: missed revenue, regulatory delays, and operational inefficiency.
FAQs
1- What is a quoting tool for medical devices?
A quoting tool automates the configuration, pricing, and proposal process for medical devices, ensuring speed and regulatory compliance.
2- How does CPQ software help with compliance?
It embeds regulatory rules directly into the quoting workflow, flagging invalid configurations and automating approvals.
3- Can it handle multilingual and regional quoting?
Yes. Tools like Cincom CPQ support multiple languages, currencies, and local regulatory requirements.
4- Will it reduce my team’s workload?
Absolutely. Sales, engineering, and legal teams spend less time on quote reviews and more time on strategic tasks.
5- Is CPQ software hard to implement?
Modern CPQ tools are cloud-based, scalable, and designed for ease of integration with your CRM and ERP systems.



